-
ORIGINAL ARTICLE
Effect of Brazilian green propolis in chronic ulcer treatment: a randomized clinical trial
Revista Brasileira de Enfermagem. 2024;77(4):e20230418
09-06-2024
Resumo
ORIGINAL ARTICLEEffect of Brazilian green propolis in chronic ulcer treatment: a randomized clinical trial
Revista Brasileira de Enfermagem. 2024;77(4):e20230418
09-06-2024DOI 10.1590/0034-7167-2023-0418
Visualizações0Ver maisABSTRACT
Objective:
to assess the effectiveness of 5% Brazilian green propolis (ointment) in individuals with chronic ulcers.
Methods:
a randomized clinical trial, developed with 40 patients randomized equally to control group (treated with essential fatty acid) and experimental group (treated with 5% green propolis) for 30 days. The outcomes of interest were sociodemographic, clinical and laboratory characteristics, lesion characteristics, such as type of tissue in the bed, presence of exudate, edge characteristics, microbial content and pain.
Results:
regarding sociodemographic, clinical and laboratory characteristics, the two groups did not show statistically significant differences. After assessment in 30 days, an effect was observed for both treated groups, but for the experimental group, greater effectiveness in terms of the type of tissue in the bed, type of exudate, edge characteristics, microbial content and pain.
Conclusion:
propolis-based ointment showed a healing effect, presenting itself as a potential tool in healing chronic ulcers.
-
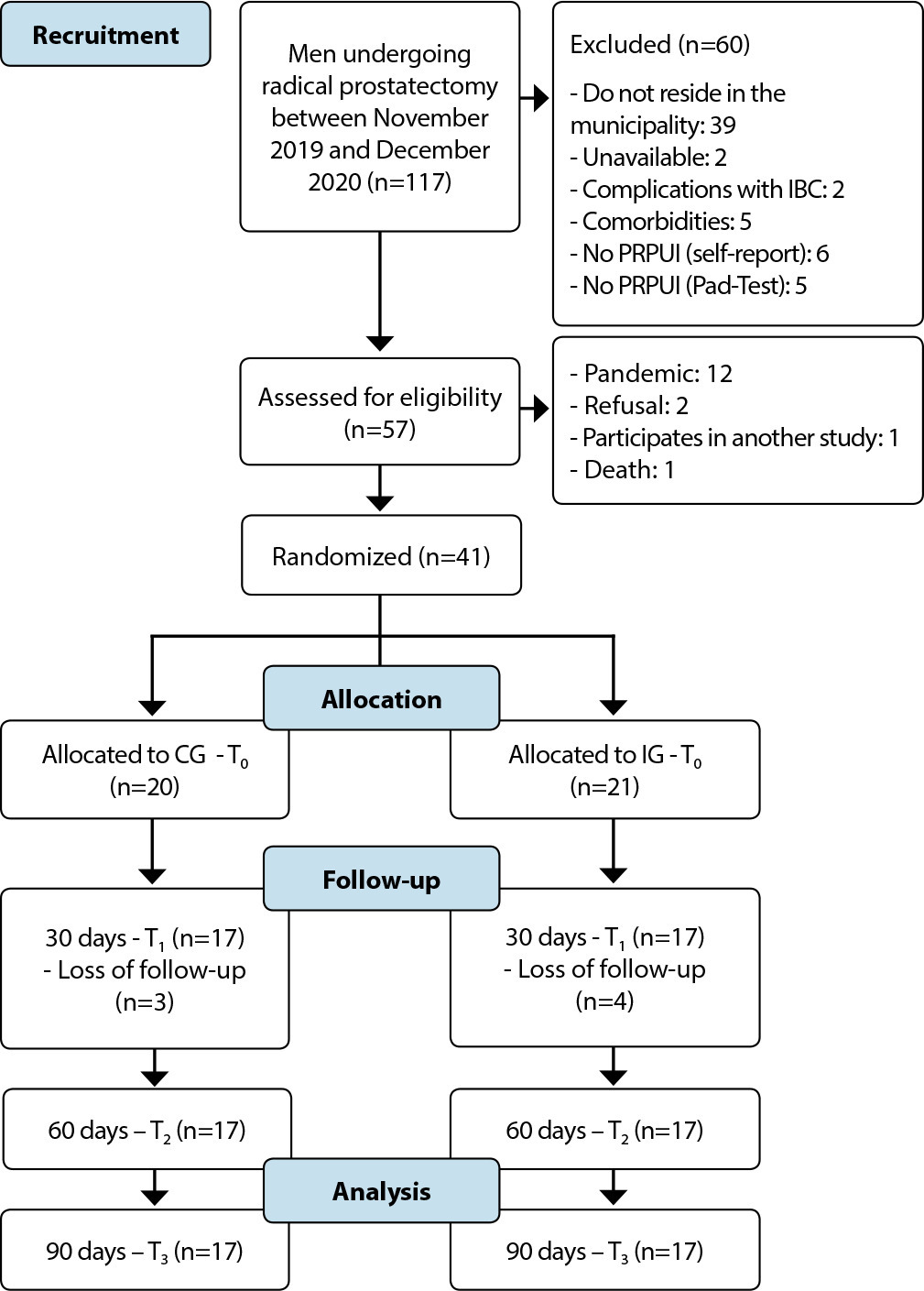
ORIGINAL ARTICLE
Cognitive-behavioral program to control lower urinary tract symptoms after radical prostatectomy: a randomized clinical trial
Revista Brasileira de Enfermagem. 2022;75(5):e20210818
07-29-2022
Resumo
ORIGINAL ARTICLECognitive-behavioral program to control lower urinary tract symptoms after radical prostatectomy: a randomized clinical trial
Revista Brasileira de Enfermagem. 2022;75(5):e20210818
07-29-2022DOI 10.1590/0034-7167-2021-0818
Visualizações0Ver maisABSTRACT
Objective:
to assess the effectiveness of a cognitive-behavioral program to control lower urinary tract symptoms after radical prostatectomy.
Methods:
a randomized clinical trial study, with 41 participants randomized into intervention (n=20) and control (n=21), for three months. The intervention group received the cognitive-behavioral program, while the control group received routine guidance from the service. Outcome variables were urinary incontinence intensity and lower urinary tract symptoms, assessed by the Pad-Test and Urinary Incontinence Scale of Radical Prostatectomy and King’s Health Questionnaire.
Results:
at the end of the study, the intervention group had a lower urinary incontinence intensity (p≤0.001), and there were less chances of presenting changes in urinary frequency (p≤0.001), urinary urgency (p≤0.001), nocturia (p=0.005), stress urinary incontinence (p≤0.001) and urge incontinence (p≤0.045).
Conclusion:
the cognitive-behavioral program was effective in reducing lower urinary tract symptoms after radical prostatectomy. Brazilian Clinical Trial Registry: RBR-3sstqg.
-
ORIGINAL ARTICLE
Effects of floral therapy on labor and birth: a randomized clinical trial
Revista Brasileira de Enfermagem. 2021;74(Suppl 6):e20210079
08-18-2021
Resumo
ORIGINAL ARTICLEEffects of floral therapy on labor and birth: a randomized clinical trial
Revista Brasileira de Enfermagem. 2021;74(Suppl 6):e20210079
08-18-2021DOI 10.1590/0034-7167-2021-0079
Visualizações0INTRODUCTION The Policy of Comprehensive Attention to Women’s Health focuses on improving obstetric care. One of the points observed in the Program of Comprehensive Attention to Women’s Health is the monitoring of pacts to reduce the rate of cesarean sections in hospitals of the Brazilian Unified Health System (SUS – Sistema Único de Saúde), rescuing […]Ver mais
-
REVIEW
Overview of clinical trial protocols for behavioral insomnia in infants
Revista Brasileira de Enfermagem. 2021;74(suppl 4):e20200769
06-28-2021
Resumo
REVIEWOverview of clinical trial protocols for behavioral insomnia in infants
Revista Brasileira de Enfermagem. 2021;74(suppl 4):e20200769
06-28-2021DOI 10.1590/0034-7167-2020-0769
Visualizações0ABSTRACT
Objective:
to describe the overview of clinical trial protocols for behavioral insomnia in infants.
Methods:
an analytical study that reviewed protocols registered with the International Clinical Trials Registry Platform between August and September 2019, aiming to identify the interventions for behavioral insomnia in infants, the comparators, the main primary, secondary outcomes and their respective measurements.
Results:
eleven protocols registered between 2004 and 2018 were included. Nurses were the main coordinators of protocols (45.5%), with proposals using educational technologies, one-to-one and online follow-up consultations. The main outcome was improvement of infant and maternal sleep patterns. Secondary outcomes were anxiety, depression, and parental sexual satisfaction. To measure them, the following were used: sleep diary (54.5%), actigraphy (45.4%), and the Pittsburgh Sleep Quality Interview (36.3%) and Extended Brief Infant Sleep Questionnaire (27.2%) were used.
Conclusion:
the protocols proposed interventions for independent sleep, aiming at quality of sleep for the whole family.
Palavras-chave: Clinical ProtocolsClinical TrialInfantSleepSleep Initiation and Maintenance DisordersVer mais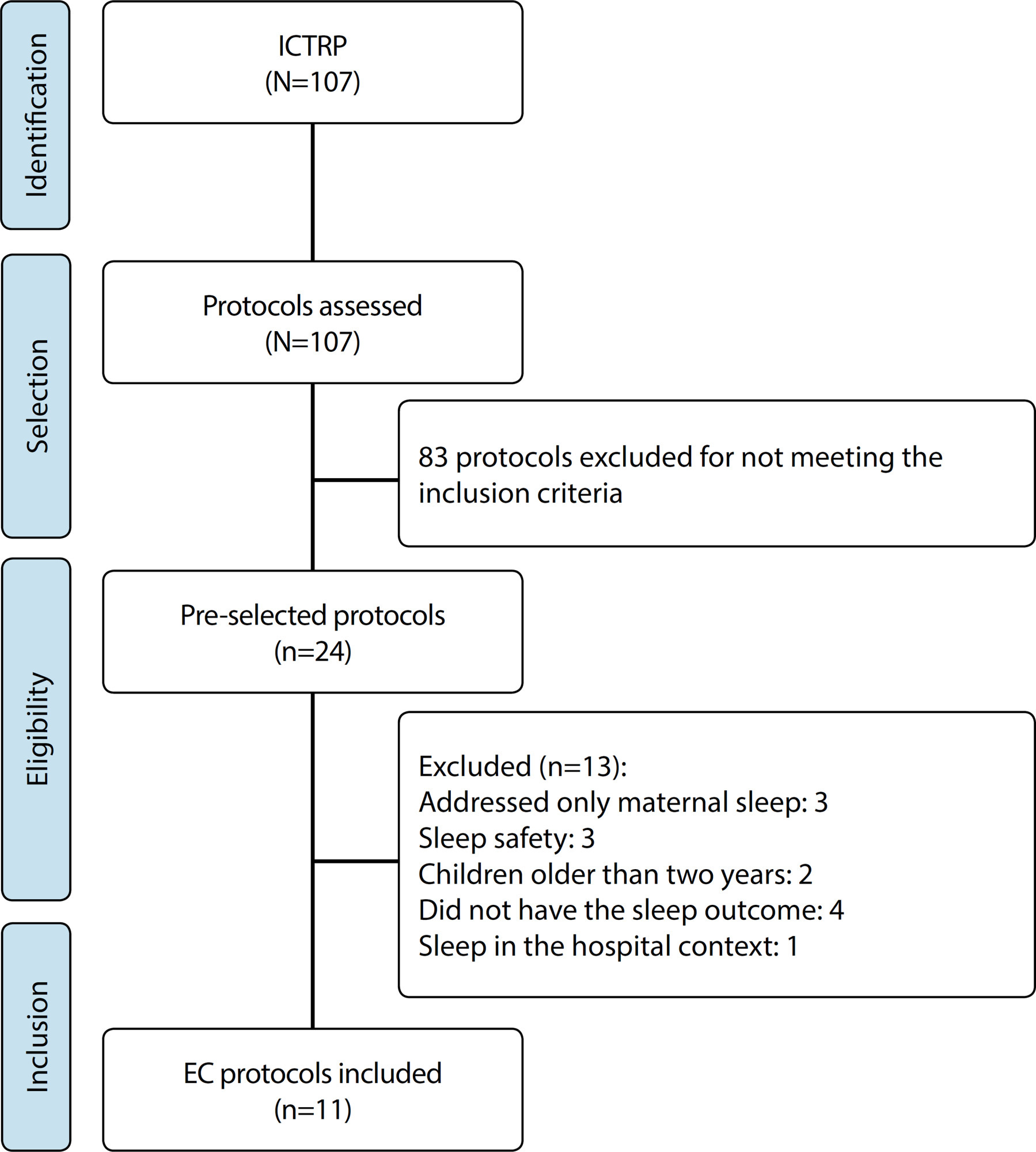
-
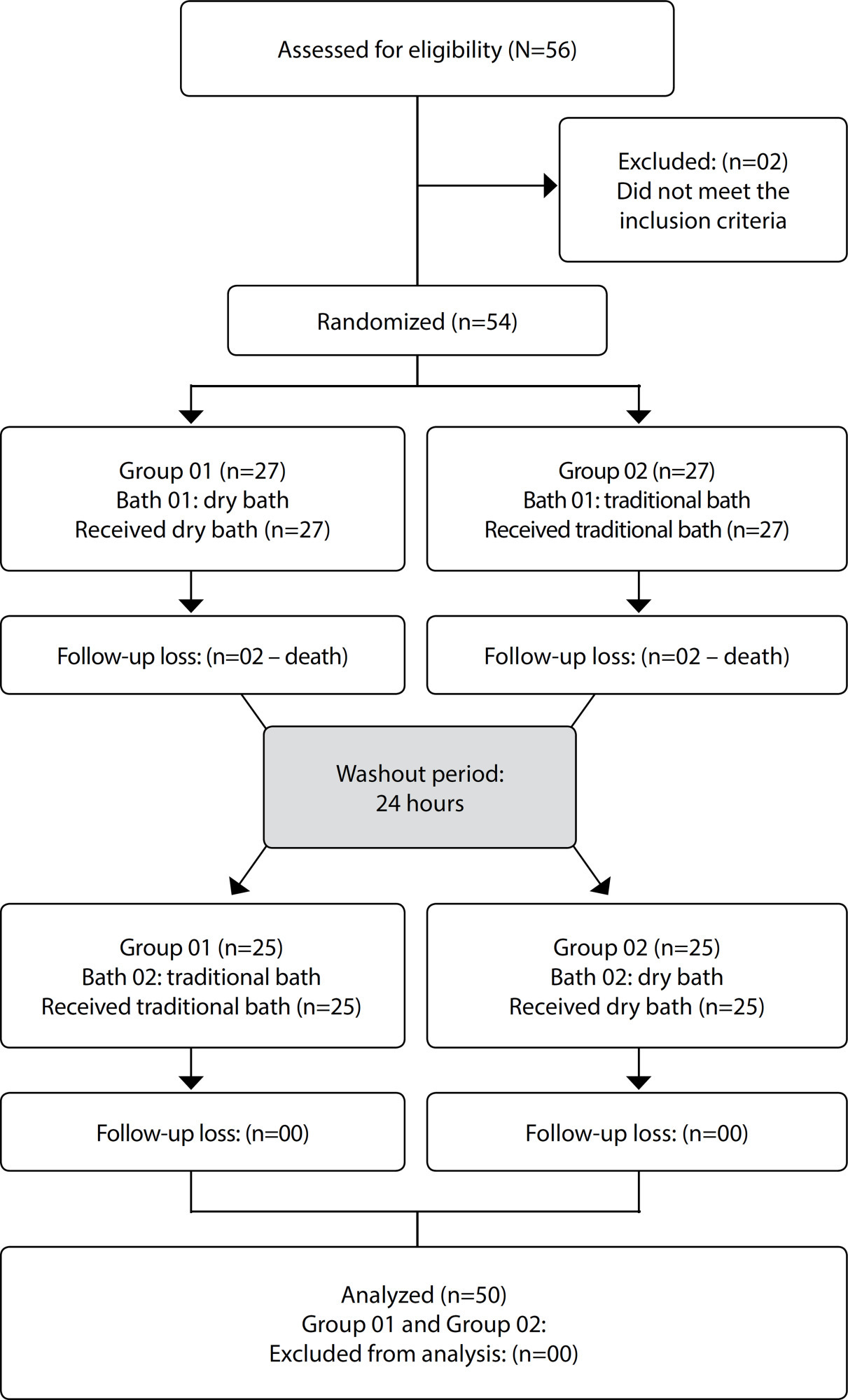
ORIGINAL ARTICLE
Changes in body temperature of critically ill patients submitted to bed bathing: a crossover clinical trial
Revista Brasileira de Enfermagem. 2021;74(2):e20200969
05-21-2021
Resumo
ORIGINAL ARTICLEChanges in body temperature of critically ill patients submitted to bed bathing: a crossover clinical trial
Revista Brasileira de Enfermagem. 2021;74(2):e20200969
05-21-2021DOI 10.1590/0034-7167-2020-0969
Visualizações0Ver maisABSTRACT
Objectives:
to compare tympanic and axillary body temperature values of critical patients before and after the traditional and dry bed bath.
Methods:
this is a randomized, open crossover clinical trial conducted with 50 adult critical patients. All patients received both types of bed bathing. The tympanic and axillary temperature values were measured at the beginning and end of the baths. The Wilcoxon test or paired Student’s t test was used.
Results:
elderly and male patients predominated. There was no significant difference between tympanic temperature medians measured during the traditional bed bath (p=0.707) and dry bath (p=0.101). Axillary temperature means reduced at the end of the baths (p=0.001), being 36.12ºC in the traditional bath and 35.92ºC in dry bath.
Conclusions:
bed bath, regardless of the method used, caused a reduction in critical patients’ axillary temperature.
-
ARTIGO ORIGINAL
Effectiveness of mobile applications in pregnant women’s adherence to prenatal consultations: randomized clinical trial
Revista Brasileira de Enfermagem. 2021;74(suppl 5):e20190599
03-15-2021
Resumo
ARTIGO ORIGINALEffectiveness of mobile applications in pregnant women’s adherence to prenatal consultations: randomized clinical trial
Revista Brasileira de Enfermagem. 2021;74(suppl 5):e20190599
03-15-2021DOI 10.1590/0034-7167-2019-0599
Visualizações0Ver maisABSTRACT
Objective:
to evaluate the effectiveness of a mobile application for cell phones in the adherence of pregnant women to prenatal consultations.
Method:
a randomized controlled clinical trial, simple-blind with two parallel groups, conducted from January to December 2018. Data collection was carried out through a structured interview at the end of the third trimester of pregnancy. For analysis, Chi-Square and Mann-Whitney tests were used. The sample consisted of 88 pregnant women from 2 Family Health Strategies in Northeast Brazil. Participants were randomized into two groups: intervention (IG), who used the application, and control (CG), who attended prenatal consultations.
Results:
pregnant women who used the application (IG) attended a greater number of consultations when compared to participants in the CG, identifying a statistical difference between the groups (p<0.05).
Conclusion:
the application showed to be an effective health technology to improve adherence to prenatal care. Brazilian Registry of Clinical Trials: RBR-74SNST.

-
ORIGINAL ARTICLE
Problematization educational intervention to promote healthy habits in elderly people with diabetes: randomized clinical trial
Revista Brasileira de Enfermagem. 2020;73(suppl 3):e20190719
10-19-2020
Resumo
ORIGINAL ARTICLEProblematization educational intervention to promote healthy habits in elderly people with diabetes: randomized clinical trial
Revista Brasileira de Enfermagem. 2020;73(suppl 3):e20190719
10-19-2020DOI 10.1590/0034-7167-2019-0719
Visualizações0Ver maisABSTRACT
Objective:
to assess the effects of a problematization educational intervention to promote healthy habits in elderly people with diabetes.
Methods:
a randomized clinical trial conducted with 202 individuals drawn for the intervention group and the control group. The intervention consisted of problematization educational activities on a monthly basis for over six months. The control group participated in conventional monitoring at the health unit. Data were collected through semi-structured interviews before and after the intervention, in addition to laboratory tests.
Results:
after the intervention, when compared to the control group, there was an increase in knowledge about the disease (p<0.001), positive attitude towards self-care (p=0.011), physical activity (p=0.020), diet variety (p=0.002), and lower consumption of oils and fats (p<0.05).
Conclusion:
the intervention performed has a beneficial effect to promote healthy habits.
-
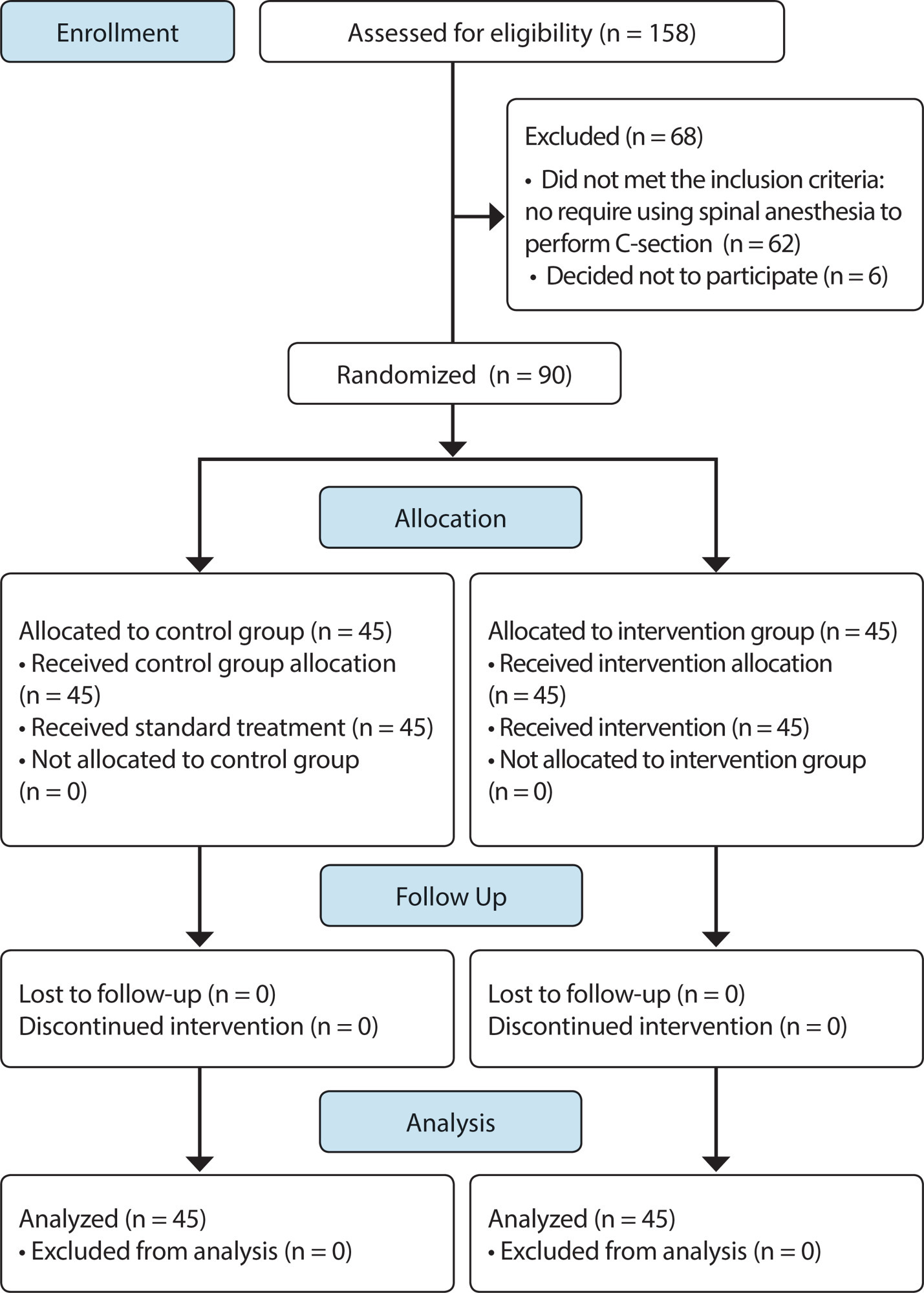
ORIGINAL ARTICLE
Educational intervention with serial album about pregnant women positioning for spinal anesthesia: randomized clinical trial
Revista Brasileira de Enfermagem. 2020;73(suppl 4):e20190878
10-05-2020
Resumo
ORIGINAL ARTICLEEducational intervention with serial album about pregnant women positioning for spinal anesthesia: randomized clinical trial
Revista Brasileira de Enfermagem. 2020;73(suppl 4):e20190878
10-05-2020DOI 10.1590/0034-7167-2019-0878
Visualizações0Ver maisABSTRACT
Objective:
To evaluate effectiveness of using educational intervention serial album to positioning pregnant women for spinal anesthesia.
Method:
Randomized clinical trial with 90 women casually assigned to control (CG) and intervention group (IG), in a maternity hospital located in Northeast region of Brazil. The primary endpoint was “achieve correct positioning to perform spinal anesthesia”; and secondary, “how number of spinal cord puncture attempts”. Effectiveness was verified using the chi-square test, Fisher’s exact test and likelihood ratio.
Results:
The positioning was correct in 37 women in each group. There was an association between women in control group remaining still, relaxing shoulders and flexing the spine; and women in intervention group should remain still and relax the shoulders; furthermore there was a statistical association achieved by correct positioning and the number of attempts to access the lumbar puncture.
Conclusion:
Educational intervention with serial album was effective and contributed to immobility and positioning of pregnant women. Brazilian Registry of Clinical Trials (RBR-3Z7SRD).